Treatment of Microcystic Adnexal Carcinoma in a 52-year-old female patient during the COVID-19 Pandemic « Contents
Dr Laura Wade, Dental Core Trainee 1 laura.wade@mbht.nhs.uk.
Mr N Hampton, Associate Specialist nick.hampton@mbht.nhs.uk
Oral & Maxillofacial Surgery, University Hospitals of Morecambe Bay Acute NHS Trust
Abstract
Introduction: Microcystic Adnexal Carcinoma (MAC) is a rare malignant sweat gland neoplasm. It is slow growing and locally aggressive but rarely metastasises. There have only been a few hundred cases described worldwide (1). During the COVID-19 pandemic, much elective surgery has been postponed, but due to the malignant nature of the lesion, this day case surgery is able to go ahead.
Methods: A 52-year-old female patient presented with a nodular lesion of the right medial eyebrow. Punch biopsy histology described a moderately differentiated squamous cell carcinoma. The lesion was excised and repaired with a local skin flap, and definitive histology reported MAC excised with 3mm margins. The case was discussed at a virtual multi-disciplinary team meeting which advised wider excision. This was undertaken with a supraclavicular full thickness skin graft to cover the defect. Both procedures were carried out as day cases; the patient was tested for COVID-19 and isolated beforehand.
Results: Histology described a highly infiltrative tumour not connecting with the overlying epidermis. It was composed of cords and nests of cells, manifesting ductular and follicular differentiation. The carcinoma invaded subcutaneous fat and perineural tissues. The cells were positive for cytokeratin AE1/AE3 and negative for BerEP4, overall favouring the diagnosis of MAC. Owing to the aggressive potential of this tumour, a wider margin was advised, the result of which was negative for malignancy.
Conclusion: MAC is a malignant tumour which often microscopically exceeds macroscopic margins, and presents clinically as a benign slow-growing lesion. This case follows the treatment of MAC during the COVID-19 pandemic.
Key Words
Microcystic Adnexal Carcinoma; COVID-19; day case
Introduction
Microcystic Adnexal Carcinoma (MAC) is a rare malignant sweat gland neoplasm, which is slow growing and rarely metastasises. Also known as syringomatous carcinoma, malignant syringoma and sclerosing sweat duct carcinoma; there have only been a few hundred cases of MAC worldwide (1). MAC generally occurs in adults aged between 50-60 years old (2), and some literature suggests it has a slight female predilection (3). Typically, MAC presents as a whitish-yellow plaque on the face, and develops a nodular appearance over time, often clinically mimicking Basal Cell Carcinoma (BCC). MAC can occasionally present with altered sensation due to perineural spread (2).
This case follows the treatment of MAC in a 52-year-old female patient as a day case at a North West General Hospital during the COVID-19 global pandemic. Due to the increasing pressures on the NHS, all elective non-cancer surgery was postponed during the height of the pandemic. However, due to the malignant nature of this lesion, surgery was able to go ahead; the patient was tested for COVID-19 and isolated prior to surgery.
Methods
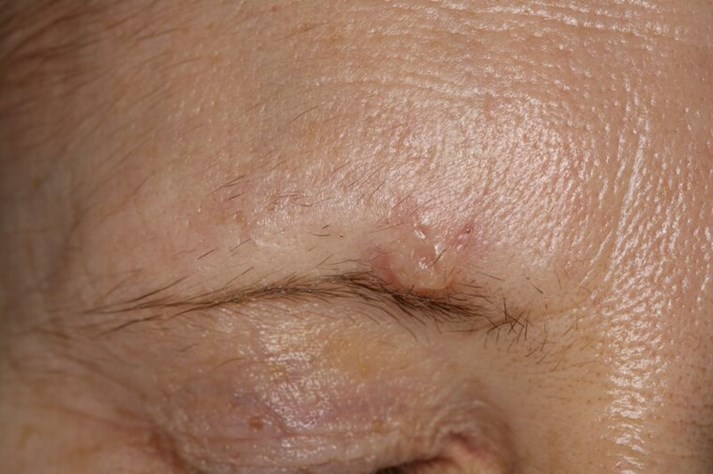
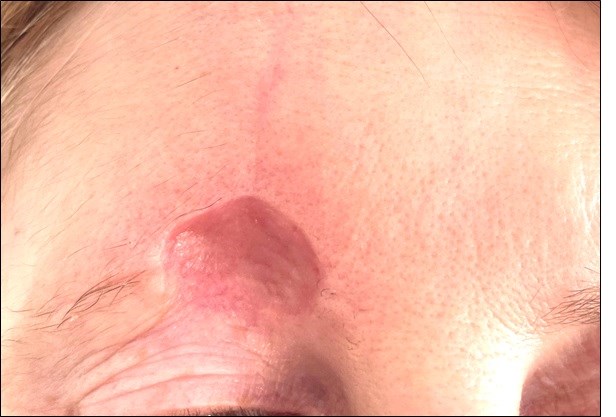
A 52-year-old female patient was referred with a three-month history of a lesion at the right medial eyebrow, which had gradually increased in size. The lesion, which on initial examination appeared benign, was 2 cm in diameter, well defined and nodular in appearance with a pearlescent hue (see Figure 1). Punch biopsy diagnosed a moderately differentiated invasive squamous cell carcinoma (SCC); invading subcutaneous and muscular tissue, at least 4.2mm deep. The patient was informed of the diagnosis and counselled about the invasive and malignant nature of the lesion. Treatment options were discussed and the patient was consented for its surgical removal under local anaesthetic. Medically, the patient was fit and well. She had never smoked and rarely consumed alcohol.
Figure 1. Photograph showing the 2cm, pearlescent nodular lesion which was well defined, with slight induration of the margins and an erythematous base in the right medial eyebrow. Clinically this appeared to be a Basal Cell Carcinoma, but punch biopsy results established an initial diagnosis of SCC. The final diagnosis after excision was Microcystic Adnexal Carcinoma.
University Hospitals of Morecambe Bay Acute NHS Healthcare Trust has three principle hospital sites. The Trust designated Westmorland General Hospital a ‘clean’ hospital where all patients who are admitted would be tested for COVID-19 and asked to isolate for a period of time before the operation. The patient agreed to this protocol, and hence her procedure was able to take place just six days after her initial consultation.
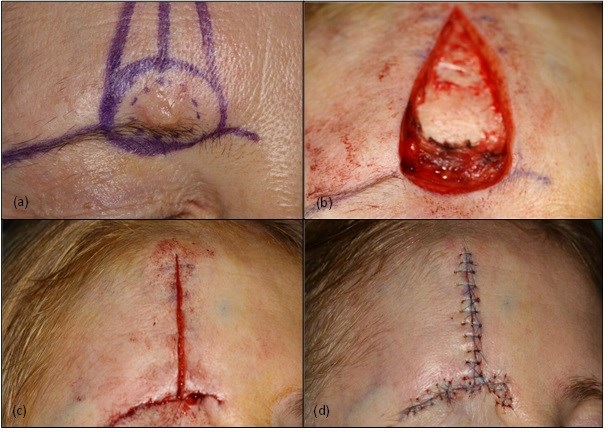
The lesion was excised under local anaesthetic, and the defect repaired with a local ‘A to inverted T’ skin flap (see Figure 2). The suspected SCC was sent to histopathology. The patient was seen a week later in the maxillofacial nurse-led dressings clinic, and the sutures were removed.
Figure 2. Photographs taken during the initial day surgery for removal of the suspected SCC and repair with an ‘A to T’ local skin flap under local anaesthetic. (a) The clinical extent of the lesion is marked (dashed line) and a 6mm margin around the lesion was to be excised. Also note the markings for the ‘A to T’ flap. Photograph (b) shows the wedge of tissue removed, including the lesion itself and excess tissue above the lesion to allow for closure with the local skin flap. The inverted ‘T’ of the flap can be visualised in photograph (c), where the skin flaps either side have been undermined to allow closure. Sutures are placed both deep and superficial, as seen in photograph (d).
Histology reported a highly infiltrative carcinoma composed of narrow cords and nests of relatively bland epidermoid cells which manifested ductular and follicular differentiation. The carcinoma invaded subcutaneous fat and showed not infrequent perineural involvement. There was no clear connection with the overlying epidermis, and the tumour cells were positive for cytokeratin AE1 and AE3, and negative for BerEP4. A DPAS stain showed focal PAS positive material within ductular spaces. The combination of these features favoured a diagnosis of Microcystic Adnexal Carcinoma, rather than the previously described SCC. The carcinoma was clear of the peripheral and deep margins by 3mm.
The case was discussed at the local skin multidisciplinary team meeting, and the regional specialist skin multi-disciplinary team meeting, both of which were held virtually due to the COVID-19 pandemic. They concluded that a wider excision should be undertaken to achieve a 1cm margin of clearance, due to the aggressive potential of the tumour. It was also recommended that the patient undergo Magnetic Resonance Imaging (MRI) of the head and neck, alongside a Computerised Tomography (CT) scan of the thorax, to assess for metastatic disease. These scans were organised, and the secondary procedure was arranged, again following the COVID-19 protocols of testing and isolation prior to day case admission.
The day case surgery was this time completed under a general anaesthetic, due to the patient’s anxieties about the procedure. Further tissue was removed from the right eyebrow, (see Figure 3), and the defect repaired with a full thickness skin graft harvested from the right side of the neck (see Figure 4), cut to size and sutured into the recipient site (see Figure 5). A Proflavine pressure dressing was sutured over the top of the graft (see Figure 6). The patient was discharged home on the day of the surgery, and seen the following week for suture removal.
Figure 3. Photographs taken during the second day case surgery under general anaesthetic. (a) The 2cm in diameter wider local excision of the previously excised MAC is outlined. Note the scars from the previous ‘A to T’ local skin flap repair. (b) The tissue is cut and (c) excised down to the level of the periosteum to ensure clearance. (d) The excised tissue, a marker stitch is placed before the specimen is sent to histopathology for analysis.
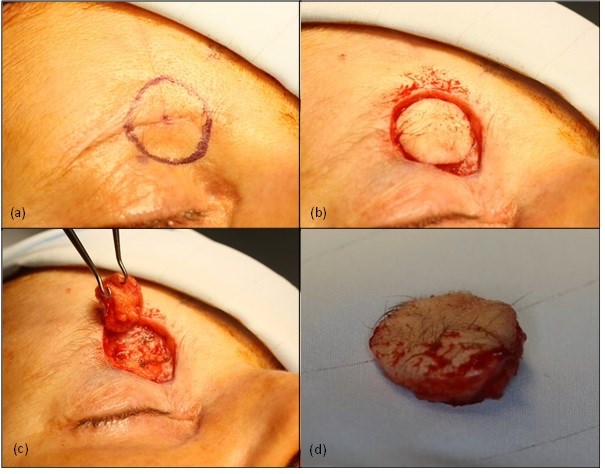
Figure 4. Photographs taken during the second day case surgery under a general anaesthetic. After the wider local excision of the MAC was undertaken (see figure 3), a full thickness skin graft was raised from the right neck. (a) The outline of the skin graft to be harvested. (b) The skin graft mid-way through excision. The skin graft composes of dermis and epidermis; any yellow fat cells are trimmed from the graft. (c) The neck wound is closed with deep and superficial sutures, and the scar is placed in a natural neck crease to reduce aesthetic disturbance.
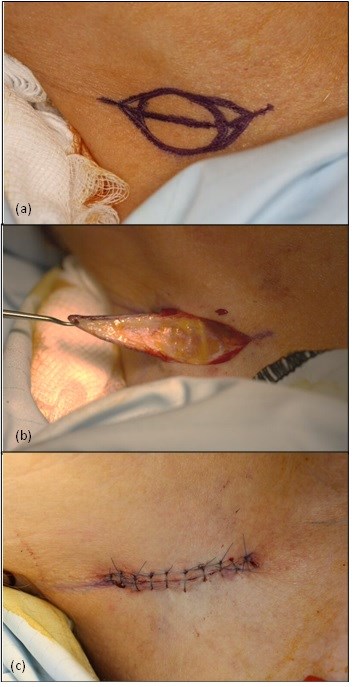
Figure 5. (a) The trimmed graft is placed into the defect, and (b) sutured in place with non-resorbable skin sutures. Quilting stitches are placed in the centre of the graft to hold the graft down to the underlying periosteum and encourage blood supply to the graft.
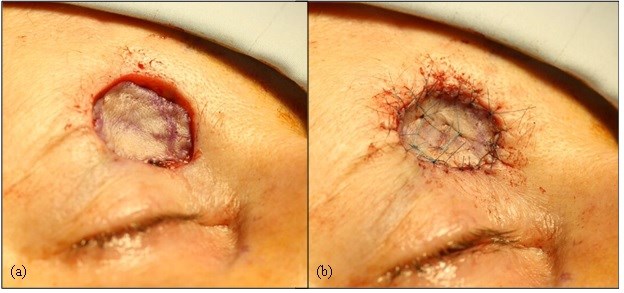
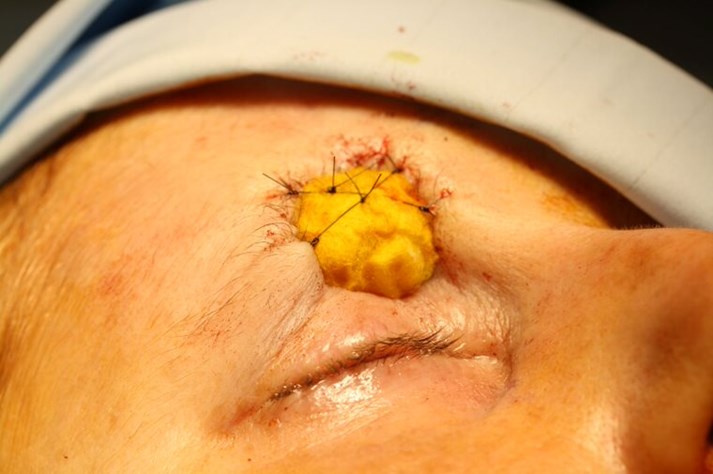
Figure 6. Final photograph of the second day case surgery. The graft is covered by cotton wool balls soaked in Proflavine bacteriostatic disinfectant and sutured into place with non-resorbable tie-over sutures.
Results
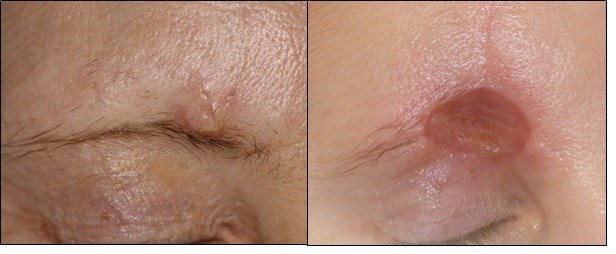
Both MRI and CT scans were clear of any metastases or lymphadenopathy, and the wider local excision demonstrated dermal scarring and a foreign body giant cell response, in keeping with the effects of previous surgery at this site. There was no evidence of residual malignancy. The surgical site has healed well (see Figure 7).
Figure 7. Photograph taken by the patient ten weeks after wider local excision of MAC and repair with full thickness skin graft from the right neck (14 weeks post initial surgery). The patient has been advised to continue to moisturise and massage the area to reduce scarring
The patient has been reassured and will now be reviewed according to our local SCC follow-up protocol, attending three-monthly appointments for the first year, four-monthly for the second year and six-monthly for the third year. The patient has been counselled regarding the importance of sun care, self-examination and urgent presentation should she be suspicious of any new or recurring lesions.
Discussion
First described by Goldstein et al in 1982 (4), MAC is a rare malignancy characterised by a pale, firm papule, plaque or nodule which is slow growing and usually asymptomatic (5). Most commonly presenting in females of the sixth decade, risk factors for MAC include but are not limited to: ultraviolet radiation, previous radiation therapy, and immunosuppressive medications (2).
MAC is often clinically overlooked or misdiagnosed, the non-sinister looking white to pink pale plaque on the face is commonly misinterpreted as a BCC, SCC, desmoplastic trichoepithelioma, trichoadenoma or syringoma (6). Originating in the pluripotent adnexal keratinocyte cell, MAC is capable of both follicular and sweat gland differentiation (7).
The misdiagnosis of MAC has also been reported, as in this case, to occur at a histopathological level after biopsy. A study in 2000 of 48 patients demonstrated that 13 (27%) had been incorrectly diagnosed at initial biopsy. The microscopic appearance of MAC is often relatively bland, especially when the biopsy is a superficial punch or shave sample, making pathological identification difficult (8).
The microscopic margins of MAC often extend far beyond the macroscopic margins, making clearance of the lesion a surgical challenge. Many surgeons choose to use Mohs micrographic surgery (MMS) to identify the extent and excise the lesion, which has been shown to be superior with respect to the number of procedures required for total excision: The 2000 study of 48 cases showed that 30% of patients with simple excision at onset will require at least one other procedure compared with as little as 0% if treated with MMS (8). The defects created by total excision with clear margins can be up to six-times the size of the initial clinical lesion (9). The extent of the defect in comparison to the original lesion can be visualised in Figure 8.
Figure 8. A comparison of the lesion before the procedures and the skin graft 4 weeks after the second day case surgery. Note the size difference between the two.
MAC is most commonly confined to skin, however there have been reports of metastasis to the lymph nodes, lungs and mediastinum (10,11), hence the need for MRI and CT imaging in this case. More commonly however is local invasion, into underlying soft tissue, bone and muscle.
Despite the low risk of metastasis, there is a considerable risk of local recurrence of MAC. Cases of recurrence have been reported up to 30 years after the primary tumour was excised (12). Tumours which have not been adequately cleared are at a greater risk of recurrence (9), hence the advised 1cm margin of clear tissue in our reported case, which was achieved following the second day case procedure.
Conclusion
MAC is a malignant, locally aggressive tumour which is commonly misdiagnosed due to its benign façade and slow growing behaviour, along with a bland histological picture. The tumour often microscopically exceeds macroscopic margins, presenting problems with complete excision and recurrence. This case follows the treatment of MAC during the COVID-19 pandemic over two-day case sessions, each carried out at in a North West Hospital Trust, with the appropriate COVID precautions and isolation procedures.
References
- Lopez, Mariela MD; Cole, Eric L. MD Microcystic Adnexal Carcinoma, Plastic and Reconstructive Surgery Global Open: November 2014 - Volume 2 - Issue 11 - p e254
- Zito PM, Mazzoni T. Microcystic Adnexal Carcinoma. [Updated 2020 Sep 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Gordon S, Fischer C, Martin A, Rosman IS, Council ML. Microcystic Adnexal Carcinoma: A Review of the Literature. Dermatol Surg. 2017 Aug;43(8):1012-1016.
- Gerall, C., Sippel, M., Yracheta, J. and Hogan, F., 2019. Microcystic Adnexal Carcinoma: A Rare, Commonly Misdiagnosed Malignancy. Military Medicine, 184(11-12), pp.948-950.
- Calderón-Castrat, X., Román-Curto, C., Santos-Briz, A. and Fernández-López, E., 2017. Microcystic adnexal carcinoma mimicking basal cell carcinoma. JAAD Case Reports, 3(6), pp.492-494.
- Zhang, L., Huang, X., Zhou, T. and Cao, H., 2020. Microcystic adnexal carcinoma: report of rare cases. Bioscience Reports, 40(1).
- Goldstein, D., Barr, R. and Cruz, D., 1982. Microcystic adnexal carcinoma: A distinct clinicopathologic entity. Cancer, 50(3), pp.566-572.
- Chiller K, Passaro D, Scheuller M, Singer M, McCalmont T, Grekin RC. Microcystic Adnexal Carcinoma: Forty-eight Cases, Their Treatment, and Their Outcome. Arch Dermatol.2000;136(11):1355–1359. doi:10.1001/archderm.136.11.1355
- Hamed, N. and Khachemoune, A., 2015. Microcystic adnexal carcinoma: A focused review and updates. Journal of Dermatology & Dermatologic Surgery, 19(2), pp.80-85.
- Ban M, Sugie S, Kamiya H, Kitajima Y. Microcystic adnexal carcinoma with lymph node metastasis. Dermatology. 2003;207(4):395-7. doi: 10.1159/000074122. PMID: 14657634.
- medscape.com. 2021. Microcystic Adnexal Carcinoma: Practice Essentials, Background, Pathophysiology. [online] Available at: <https://emedicine.medscape.com/article/1101894-overview> [Accessed 24 February 2021].
- Lupton, G., 1986. Microcystic adnexal carcinoma. Report of a case with 30-year follow-up. Archives of Dermatology, 122(3), pp.286-289.
Download this article as PDF here: https://appconnect.daysurgeryuk.net/media/51041/312-treatment-of-microcystic-adnexal-carcinoma.pdf