Pre-emptive oral gabapentin and effects on post-operative pain relief after day case gynaecological procedures « Contents
Emeka Ibem Kalu, Senior registrar, Olubusola Alagbe-Briggs, Consultant, Longinus Ndubuisi Ebirim, Consultant
Department of Anaesthesiology, University of Port Harcourt Teaching Hospital, Port Harcourt, Rivers State, Nigeria
Corresponding author: Dr L N Ebirim, Department of Anaesthesiology, University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria . Tel: +2348033384198 . Email: longinus.ebirim@uniport.edu.ng
Abstract
Introduction: Pre-emptive administration of oral gabapentin may cause a reduction in dose and side effects of analgesic agents needed to achieve adequate postoperative pain relief. This study was therefore aimed at determining if pre-emptive oral gabapentin can attenuate post-operative pain following day case gynaecological surgeries performed under general anaesthesia.
Methods: Fifty-six ASA I or II patients scheduled for day case gynaecological operations were recruited into this prospective, double-blinded study after obtaining informed consent from them. They were randomly allocated into two groups (GG and PG) of 28 patients each, to receive either 300mg of oral Gabapentin (GG) or Placebo capsules (PG) 2 hours before surgery.
The outcome assessed included time to first request for postoperative analgesia, pain intensity, analgesic consumption in the postoperative period before discharge as well as possible side effects.
Results: The time for first analgesic request was significantly prolonged in the Gabapentin group compared to the Placebo group. (p= 0.007). The mean VAS score was significantly lower in the GG than in the PG. (p= 0.001). The mean total dose of analgesic consumed during the study was significantly lower in the GG than in the PG, (p= 0.004). All patients in both groups were discharged between 8 and 10 hours after surgery. The proportions of patients that experienced nausea, vomiting and dizziness were similar in both groups.
Conclusion: Gabapentin 300 mg given orally 2 hours prior to surgery decreased postoperative analgesic requirements and prolonged the time to first rescue analgesic request with minimal side effects.
Keywords: Pre-emptive gabapentin, post-operative analgesia,
Introduction
Pain is defined by the International Association for the Study of Pain (IASP) as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or that can be described in terms of such damage.1
Pain management in day case surgical patients is a major concern to the surgeon and the anaesthetist and a multi-modal approach is ideal to achieve adequate pain control in this group of patients.2
Inadequately treated postoperative pain can lead to patient discomfort, sleep deprivation, prolonged hospital stays, unanticipated hospital readmission and increased costs.3
A day case patient is one who is admitted for a surgical procedure or investigation and discharged within 24 hours following adequate recovery from anaesthesia.4 Discharge criteria for day case patients include ability to sit unaided, achieve good pain relief with oral analgesics, walk in a straight line, ingest fluid and food without nausea and vomiting, as well as void urine.5
A study has shown that under-treatment of pain is common after day case surgery and about 30 - 40% of discharged out-patients may suffer from moderate to severe pain during the first 24 – 48 hours.6
Therefore, optimal post-operative pain control for day-case surgeries should be achieved using drugs with minimal side-effects that facilitate early recovery. For this reason, several pharmacological agents have been employed to control pain in day case patients.7
Opioids have been given by various routes for this purpose. However, the side effects of opioids like nausea, vomiting, and respiratory depression limit their usage and can delay discharge.8 Non-steroidal anti-inflammatory drugs are also being employed especially in the immediate post-operative periods but concerns are raised about their side effects like inhibition of cyclooxygenase which may lead to acute gastritis, stress ulcers and bleeding.9
Different mechanisms are involved in post-operative pain, such as sensitivity to nociceptors and inflammatory component. The process is created by pain receptors (nociceptors) which are sensitive to mechanical, chemical and heat stimulation. These generate nerve messages throughout the brain and spinal cord, leading to pain.10
Therefore, multimodal analgesic techniques utilizing a number of drugs acting on different analgesic mechanisms are becoming increasingly popular.11 Also, the concept of preventing the pain before it starts by desensitizing the central nervous system is being explored with the use of pre-emptive analgesia.
Some studies have been carried out on the use of oral gabapentin for pre-emptive analgesia,12,13 Gabapentin was found to have enhanced the analgesic effect of other pain-relieving agents as evidenced by increase in time to first request for analgesic postoperatively, there was also a significant reduction in total analgesic consumption in the first 24hours.
Commonly reported side effects of gabapentin include dizziness, sedation, ataxia, blurred vision and irritability.12 However, most of the adverse effects following the use of oral gabapentin are dose dependent. Hence, 300mg of oral gabapentin does not cause significant side effects as shown by Montazeri et al13
Some studies have shown that single dose 600mg as well as 300mg oral gabapentin attenuated post-operative pain14, 15 Few of these studies have been done in this sub-region especially in patients undergoing day case gynaecological surgeries. Therefore, oral gabapentin was evaluated for its pre-emptive analgesic efficacy on post-operative pain in patients undergoing day case gynaecological surgeries in this study.
Methods
This randomized, double-blinded, prospective study was carried out at the University of Port Harcourt Teaching Hospital (UPTH) from January 2017–June 2017.
The study population was drawn from patients aged between 20-50 years of ASA physical status I and II, scheduled for day case gynaecological surgeries under general anaesthesia. Excluded from the study were patients who refused to give consent, those who were morbidly obese and those with known allergy to gabapentin, opioids or tramadol. Also excluded were patients on gabapentin or analgesic usage 24 hours prior to surgery, those with chronic pain and those with history of seizures or psychiatric disorder.
Sample size calculation16 showed that a minimum of 50 participants were required for the study. Allowing 10% loss to protocol violation (attrition), a total of 56 patients approximately, 28 for each group were therefore entered for the study. Group I received 300mg of oral gabapentin two hours prior to surgery. Group II received oral placebo two hours prior to surgery.
Study protocol
Ethical clearance was obtained from the institutional Ethics and Research committee and. eligible patients were identified during the preoperative evaluation. The study was explained to them in a language they understood. Written consent was obtained from them. The patients were clinically assessed and fitness for the study verified. The method of pain assessment: using visual analogue scale (VAS) was explained to the patients. They were educated on the VAS which is a single line of 10cm with words at both ends: “no pain” and “worst pain” imaginable. Patients observed 6hours fast before the surgery.
Randomization
Using a balloting technique, with coded papers inside an envelope, the patients were randomly allocated into two groups, Group 1(GG) and Group 2 (PG). Patients in GG (n=28) received 300mg oral gabapentin (Neurontin) while those in PG (n=28) received oral placebo capsule (Astymin) all two hours before surgery.
The researcher who carried out the general anaesthesia procedure was blinded to the content of the envelope and the drug preparation (Neurontin capsule 300mg by Pfizer and matching placebo capsule, an inactive formulation similar to gabapentin in appearance (Astymin).
Study procedure
On the patient's arrival at the operating room a multi-parameter monitor's (Dash 4000; GE Medical System Information Technology International, Wisconsin USA) probes and cuff were attached. Baseline vital signs including heart rate (HR), systolic blood pressure (SPB), diastolic blood pressure (DBP), mean arterial blood pressure (MAP), arterial oxygen saturation (SPO2) and temperature (T°C) were taken and recorded. Electrocardiogram (ECG) monitoring was also established. Intravenous access was secured with 18-gauge cannula in the dorsum of the left hand and each patient was given 0.01mg/kg of midazolam, 0.004mg/kg of glycopyrrolate and 1mg/kg of tramadol pre-induction, all intravenously.
The patients were pre-oxygenated for 5 minutes and anaesthesia was induced with intravenous propofol 1.5mg/kg, while intravenous suxamethonium chloride 1.5mg/kg was given for muscle paralysis. Following adequate muscle relaxation, laryngoscopy was done, an appropriately sized cuffed orotracheal tube was passed, the cuff was inflated and the tube connected to the breathing circuit and anaesthetic machine. Correct tube placement was confirmed with auscultation and capnography. Anaesthesia was maintained with isoflurane (1% -1.5%) in 100% oxygen and muscle paralysis maintained with intravenous atracurium 0.5mg/kg. Non-invasive Blood Pressure, mean arterial Blood Pressure, Heart Rate and Arterial Oxygen Saturation were noted every 5minutes until the surgery was over. Fluid maintenance was achieved with 0.9% normal saline. All intravenous fluids were warmed to 37˚C to reduce the risk of hypothermia and shivering. At the end of the surgery, the residual muscle paralysis was reversed with intravenous neostigmine 0.05mg/kg and glycopyrrolate 0.008mg/kg to obtund its muscarinic effects. The trachea was extubated when the patients became conscious and able to obey commands. The duration of the surgery which is the time from knife on skin to the end of surgery (time of last stitch) was noted. Monitoring was continued post-operatively at the post- anaesthesia care unit where close monitoring of physiologic parameters; heart rate, blood pressure, temperature and respiratory rate was continued.
Patient's level of pain was assessed using the VAS. This was done when patient had recovered fully from anaesthesia as follows, when patient is conscious and obeys command (0hr), hourly for the first 2hours, and two-hourly for the next 4hours post-operatively. At the pain score of more than 3cm, rescue analgesia with intravenous tramadol 1mg/kg was given. Analgesia was maintained at home with 100mg oral tramadol given as required. The time for the first request for analgesia was noted. Occurrence of nausea, vomiting, dizziness, sedation, respiratory depression and other untoward effects among the treatment groups was also noted and recorded while patient was still on the ward and before discharge from hospital.
Patients with minimal or no side effects were discharged home between 8hrs to10hrs post-operatively, none of the patients reported any side effects at home.
The primary outcome was time to request for the first analgesia defined as the time interval from tracheal extubation to time of first analgesic request.
The secondary outcomes were: pain intensity assessed with VAS, the total dose of tramadol given within the first 24 hours after surgery and the side effects documented in the recovery room when patient was fully awake.
- Sedation was assessed in the PACU when the patient had recovered fully from general anaesthesia and graded according to the Ramsay sedation scale17 Sedation was reported as present with scores
- Respiratory depression was said to have occurred in patients in whom respiratory rate decreased to < 8 breaths per minute.
- Dizziness – This was assessed before patient was discharged from the ward using a four-point Likert verbal scale (none, mild, moderate and severe)
- Nausea and vomiting – This was graded on a four point ordinal scale (0–no nausea or vomiting.
1 = nausea but no vomiting. 2 = both nausea and vomiting present. 3 = more than two episodes of vomiting in 30 minutes
Data collection and analysis
Socio-demographic data and peri-operative events were recorded using the proforma.
Data analysis was done with SPSS Version 21. Data presentation included tables and charts. Data were tested for normality using Kolmogorov-Smirnov statistics. Normally distributed variables were summarized using means and standard deviation, and differences in means compared using independent t test while non-normally distributed variables were summarized as medians and non-parametric test of Mann-Whitney U test employed for comparing differences. Categorical variables were compared using Fisher’s exact test or Chi-square as appropriate. A P-value of <0.05 was considered statistically significant.
Results
A total of fifty-six females, aged between 20 and 50 years of ASA physical status I or II were enrolled into this study, with 28 patients in each group. Group I was the gabapentin group while group II was the placebo group. Fifty-six patients completed the study.
Both groups were comparable in terms of age, weight, height, BMI, and the duration of surgery as shown in Table I. The mean age of the patients in the gabapentin group was 35.18±5.76 years while in the placebo group, it was 33.75±5.26 years (p=0.337). The mean weight of patients in the gabapentin and placebo groups were 65.14±5.37kg and 64.46±4.95kg respectively (p=0.625). The mean height in the gabapentin group was 1.63±0.05 metres while in the placebo group, it was 1.64±0.06metres (p=0.890). The mean duration of surgery in the gabapentin group was 56.86±7.18minutes while it was 55.86±7.76minutes in the placebo group, (p=0.619).
Table I. Comparison of mean socio-demographic characteristics across groups in the study
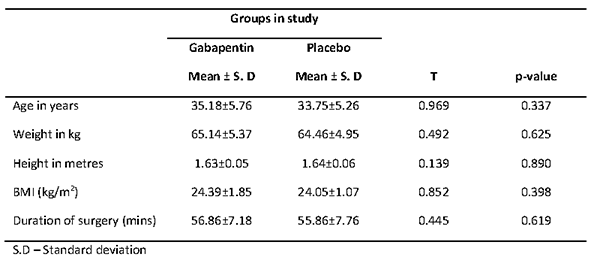
Table II shows the ASA classification and type of surgery across the groups in the study. Majority of the patients in both gabapentin (75.0%) and placebo (82.1%) groups were in ASA physical status I. There were no significant differences in proportions of ASA classification by the groups in the study (p=0.5148). The commonest surgery was adhesiolysis in both gabapentin (32.1%) and placebo (28.5%) groups. This was followed by EUA (25.0%) in the gabapentin group and suction evacuation (25.0%) in the control group. There were no significant differences in the proportions of type of surgery across the gabapentin and placebo groups (p=0.810).
Table II. ASA classification and type of surgery across groups in the study.
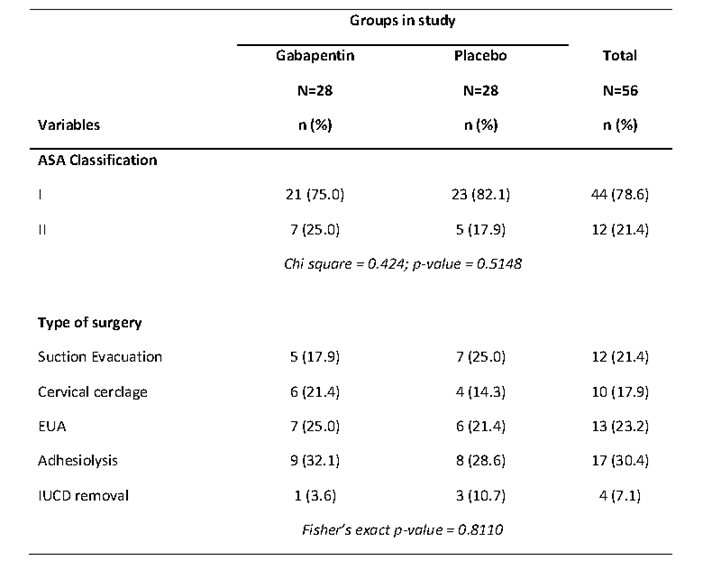
The intra-operative haemodynamic variables were stable and similar in both groups as shown in Figures 1 to 3. The differences in haemodynamic variables across groups were not significant (p>0.05). The oxygen saturation ranged between 96 to 100 % in both groups.
Figure 1: Line graph showing the mean systolic and mean diastolic blood pressure values of the
groups in the study during the intra-operative period.
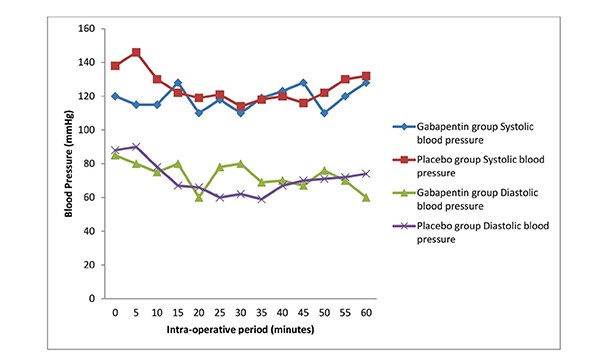
Figure 2: Line graph showing the mean heart rate values of the groups in the study during
the intra-operative period.
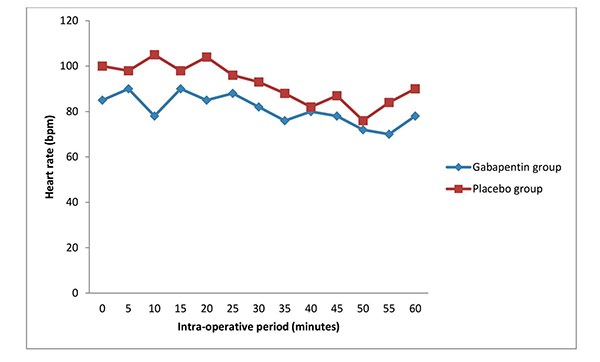
Figure 3: Line graph showing the mean SP02 of the groups in the study during the intra-operative period.
Table III (a) shows the comparison of the pain scores across the groups during the post-operative period. At time 0min (full recovery from anaesthesia and obeys command), the pain score was lower in the gabapentin group (mean score of 2.37) in comparison to the placebo group (mean score of 3.33). This difference in the pain scores was significant (p<0.05). The pain scores were also lower in the gabapentin group compared to the placebo group at 60mins, 120mins, 240mins, 360mins and 480mins respectively. The differences in the pain scores between the two groups were statistically significant (p<0.05) in most of the follow up postoperative periods.
Table III (a). Comparison of pain scores across groups in the study during the post-operative period.
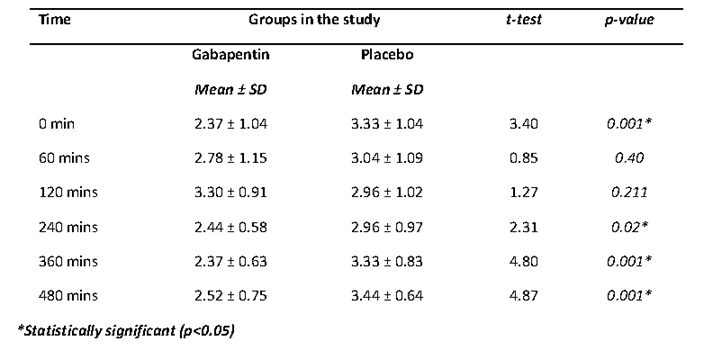
Table IV shows that the mean time of first analgesic request was prolonged in the gabapentin group (134.32±3.51minutes) compared to the placebo group (77.79±4.79minutes). The observed difference was statistically significant (p= 0.0001).
Table IV. Comparison of mean time to first request of rescue analgesia across groups in the study.
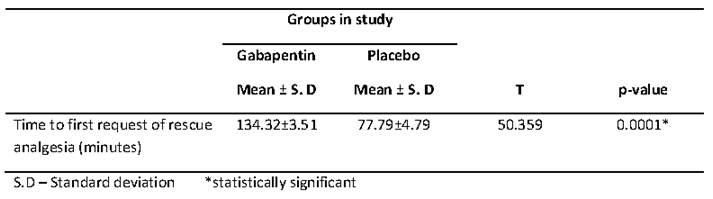
Figure 4 shows an error bar of the mean amount of opioids consumed by gabapentin and placebo groups. The mean total opioids (tramadol) consumed during the study was lower in the gabapentin group (130.8±6.4mg) in comparison to the placebo group (136.2±5.3mg). This difference in means was statistically significant (p= 0.004).
Figure 4. Error bar showing the mean amount of opioids consumed in gabapentin and placebo
groups in the study.
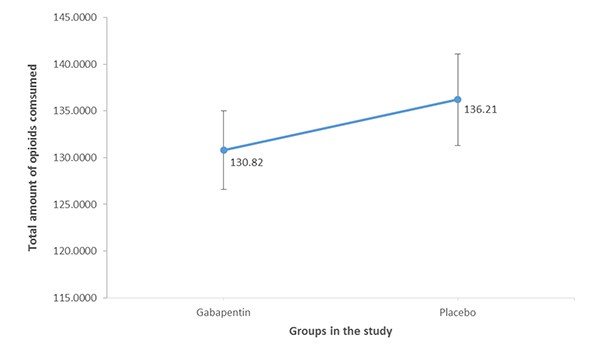
Figure 5 shows the frequency of side effects across the groups in the study. The frequency of nausea was higher in the gabapentin group (14.2%) when compared to the placebo group (10.7%). Higher proportion of the patients in the gabapentin had vomiting in comparison to the placebo group (7.1% vs 3.8%). All the patients with vomiting were treated with intravenous metoclopramide 10 mg and the vomiting resolved. Few patients complained of headache which resolved even before intervention within twenty minutes (1 patient in gabapentin group and 2 patients in the placebo group). There was also complaint of dizziness, though transient while patient was on the ward prior to discharge. The frequencies of dizziness in the gabapentin and placebo groups were 7.1% and 10.7% respectively. Fatigue was reported among 2 patients in the placebo group (7.1%), and none in the gabapentin group (0.0%).
Fig 5: Multiple bar charts showing the absolute and relative frequency of the side effects observed
in gabapentin and placebo groups.
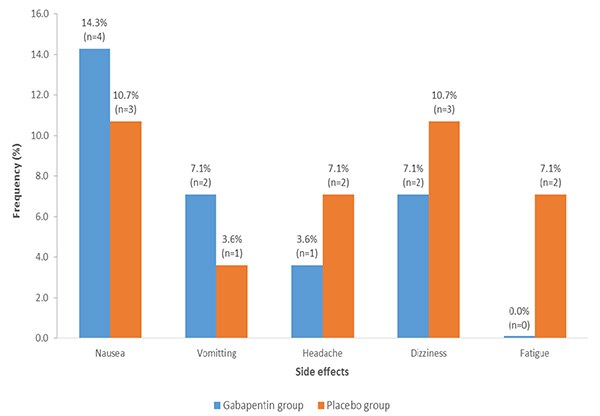
All patients in both groups were discharged between 8 and 10 hours after surgery.
Table V shows the level of satisfaction between the two groups. In the gabapentin group, 32.1% (nine of the patients) reported excellent level of satisfaction as compared to 21.4% (six of the patients) in the placebo group The proportions of patients that reported their level of satisfaction as very good were 46.4% (13 patients) in the gabapentin group while it was 39.3% (11 patients) in the placebo group. The proportions of patients that reported their level of satisfaction as being good were 17.9% (five patients) and 32.1% (nine patients) in the gabapentin and placebo groups respectively. In the gabapentin group, 3.6% of the patients reported their level of satisfaction as being fair while in the placebo group, it was 7.1 % of the patients. There was no significant difference in the proportions of level of satisfaction across groups in the study (p = 0.5321).
Discussion
This study confirms that the oral administration of 300mg gabapentin prior to day case gynaecological procedures provided improved post-operative analgesic effect and prolonged the time to first request for analgesia with minimal side effects. It also demonstrated a reduction in the post-operative pain scores at different time points, and reduction in the total amount of analgesics required 24hours post-operatively in the gabapentin group when compared with the placebo group. These findings showed that pre-emptive oral gabapentin enhanced post-operative analgesia.
Both groups were comparable in terms of age, weight, height, intra-operative haemodynamic variables and duration of surgery. The ages and surgery durations were comparable in the studies done by Mardani-Kivi et al18 and Bafna et al14. In this study, the trends of heart rate mean systolic and diastolic blood pressures were similar between the two groups. The initial high values of pulse rates and systolic blood pressures noted in this study were probably due to anxiety, the values normalized as the surgery progressed. The finding of no significant haemodynamic variation between both groups in the study is similar to that of Bafna et al14, which revealed that gabapentin had no significant impact on the haemodynamic variables between the study group and the placebo group.
Various studies have shown that under treatment of pain following surgery is common; more than 30% of discharged post-operative patients suffer from moderate to severe pain within the first 48hours6 Luscombe et al19 conducted a study to compare the effect of parecoxib and placebo for pain management following day case gynaecological surgeries. The result showed that the study group had mild to moderate pain though the pain scores were lower in the study group compared to the placebo group.
The study by Liza et al17 titled; pain management in day case surgery, also noted that only 60% of patients undergoing minor gynaecological procedures including laparoscopic surgeries reported a satisfactory pain relief post operatively, more than 30% of the study population had mild to moderate pain post operatively which is similar to the findings in this study. To this end, the need for an effective pain control following minor gynaecological surgeries similar to the procedures in this study has been well demonstrated by the studies of Luscombe et al19 and Liza et al.17
This study showed that the pain scores using VAS were lower in the gabapentin group compared to the placebo group, this is similar to the findings in various studies done by Aryal et al20, Butt et al21, Dirks et al15 and Montazeri et al13 where the VAS scores were lower in the study groups than the placebo groups showing more evidence of the post-operative analgesic effect of gabapentin. In this study, the difference in the VAS scores between the two groups at the 10th hour post-operative period was not statistically significant, this may be attributed to the half-life of gabapentin which is 5-7hours.12 However, the exact median VAS score at the 10th hour post-operatively was lower in the gabapentin group compared to the placebo group. There was prolonged time to first analgesic request in the gabapentin group in comparison to the placebo group, which is in keeping with the study by Aryal et al20, which sought to evaluate the effect of gabapentin on post-operative analgesia with epidural morphine after abdominal hysterectomy. The finding of the index study also compares with studies by Hema et al22, Montazeri et al13 and Rajendran et al23 where there were findings of significant prolonged time to the request of first analgesia among gabapentin groups in comparison to groups without gabapentin. These studies along with present study further demonstrate that pre-emptive administration of oral gabapentin enhances post-operative analgesia.
This study revealed that the total analgesics consumed within 8hours post-operatively was significantly lower in the gabapentin group compared to the placebo group, which is consistent with findings by Dirks et al,15 who reported that there was 50% reduction in the morphine consumption 24 hour post-operatively in the gabapentin group. The findings by Aryal et al20, Mardani-Kivi et al18, Rajendran et al23 and Bafna et al14 were similar to that of this study where the reduction in the post-operative opioid consumption was significantly lower in the gabapentin group compared to the placebo groups.
The use of opioid analgesics for post-operative pain management has continued to attract concerns in view of the associated side effects.7 Therefore, the analgesic potentials of gabapentin for acute pain as demonstrated by the outlined studies23,18,14,20 and index study accounts for the reduction in the quantity of opioids to be consumed post-operatively with inherent reduction of the adverse effects of opioids.
Despite these studies23,18,14,20 that demonstrated a reduction in the consumption of opioids following pre-operative oral administration of gabapentin, a study by Panah et al24 refuted the finding as the authors noted that there was no significant decline in post-operative morphine consumption among the gabapentin group, compared to the placebo group. The study by Panah et al24 was done under spinal anaesthesia technique with bupivacaine as the local anesthetic agent.
The duration of action of bupivacaine is 2-8 hours25, this might have accounted for the contradictory findings by Panah et al24 amongst other factors. There were no side effects of gabapentin observed in the study by Panah et al24 when compared to this study where nausea, vomiting and dizziness were noted.
There were no significant differences in side effects observed between gabapentin and placebo groups in this present study. This is in contrast to the study by Jabalameli et al26 which recorded higher incidence of nausea and vomiting in the placebo group. This could have resulted from the anaesthetic technique used as hypotension following spinal anaesthesia can increase the incidence of nausea and vomiting. This present study was done under general anaesthesia thereby avoiding such a conflicting situation.
To the contrary also is the study by Mardani-kivi et al18 where the incidence of vomiting was similar in the three groups despite similar mode of anaesthesia as in this study. Cruz et al27 in a pilot study confirmed the role of gabapentin in the prevention and treatment of nausea and vomiting. The increase in the incidence of vomiting in this study may be due to the combined effect of gabapentin and tramadol drug-drug interaction (pharmacodynamics). It has also been found that vomiting is an uncommon side effect of gabapentin especially in children.28
Other side effects which the patients complained of were headache (1 in gabapentin group and 2 in placebo group) and dizziness, which is one of the commonest side effects of gabapentin28 (2 in gabapentin group and 3 in placebo group). Similar side effects were reported in a study by Bafna et al14 in which there were also no significant difference between the two groups in terms of side effects. The findings of this present study are similar to that of Dirks et al15 and Mardani-Kivi et al18 in respect to the observed side effects as there were no significant differences between the study groups and the placebo groups. Similarly, the studies by Panah et al24 and Rajendran et al23 revealed no significant side effects in both groups. This could have been as a result of the anaesthetic techniques used in the studies23,24. The studies23,24 were done under spinal anaesthesia which involved the use of only local anaesthetic agent unlike general anaesthesia that involves multiple drug usage.
This study revealed that no patient reported a low level of satisfaction with the use of gabapentin for post-operative analgesia. This is in keeping with the study by Turan et al.29 which noted that patient satisfaction with post-operative pain management at 24hours was better in the gabapentin group compared to the placebo group. In this study, rescue analgesia was given from pain score above 3 as against pain score of 7 in the study of Rajendran et al23, this may have accounted for the better patient satisfaction found in this study compared to that of Rajendran et al23. At a pain score of 7 which is equivalent to moderate pain, the patient is already in painful distress, anxious and agitated hence poor patient satisfaction is achieved.
The study by Parikh et al30 combined tramadol and diclofenac for rescue analgesia, diclofenac being a Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) could cause gastritis, peptic ulcer disease and renal impairment. Hence, tramadol was carefully chosen in this study for rescue analgesia to avoid possible complications of NSAIDS which could occur especially in fasted patients.
The ASA physical status of every patient was clearly stated in this study, whereas the study by Dirks et al15 did not specify the ASA physical status of the study population. Pain perception and interpretation may be affected by patient ASA classification as an ASA V (moribund patient) may have altered sensorium and, may not be able to interpret pain perception accurately. This further authenticates the findings of this study when compared to that of Dirks et al15.
The recruitment of only female patients in this study may limit applicability of its findings to the female gender as. it has been found that there is a variation in pain perception in males and females, with females reporting higher pain intensity31. Pain assessment was carried out more than four times in this study unlike the study by Mardani-Kivi et al18 where pain was assessed only at the 6th and 24th hours after surgery, two values may not effectively represent the pain distribution of the study population.
Amanor-Boadu et al32 also carried out a study on patients undergoing gynaecological procedures to determine if ketamine has a pre-emptive analgesic effect. That study also had a homogenous sample population similar to this one. However, their patients had major gynaecological surgeries which could cause more intense post-operative pain due to more tissue damage in contrast to this study which recruited patients for day case procedures (minor surgeries). Their conclusion that pre-emptive analgesic effect could not be demonstrated could also be due to the drug they studied.
The results from this present study demonstrated the ability of preemptive analgesic intervention using 300mg of gabapentin to attenuate post-operative pain scores, decrease supplemental postoperative analgesic requirements, and prolong time to first rescue analgesic request. The pre-emptive analgesia involves preventing the pain before it starts by desensitizing the central nervous system.
Gabapentin is readily available as an over the counter prescription drug, and affordable by the average Nigerian patient. Gabapentin is less likely to cause addiction when taken in normal doses. Within the confines of this study, the side effects are mild and insignificant when compared to that of the placebo group. The benefits in the use of pre-emptive gabapentin are many. These include economic benefits as total amount of analgesics given to control post-operative pain is reduced, improved patient satisfaction due to better quality of post-operative pain control, reduced side effects of opioids as less amount is used for pain control. Others include early ambulation as well as reduced hospital stay thereby reducing the incidence of deep vein thrombosis (DVT) and thromboembolism as a result of prolonged immobilization. This is very relevant in the day case surgeries where opioids especially the long acting ones are not ideal, due to associated side effects like nausea and vomiting which delay the discharge of the patients from the hospital.
Limitations
The recruited patients were not evaluated for visual impairment which affects the accuracy of Visual Analogue Scale used for pain intensity assessment
There was a language barrier while administering the tools for pain intensity assessment. However, the use of an interpreter was of importance in ensuring accurate data collection.
The surgical procedures in this study were not homogenous as different tissues handling may have different degree of pain receptors hence, varying pain perception. However, the surgeries were restricted to gynaecological procedures only.
Conclusion
This study has demonstrated that oral administration of 300 mg of gabapentin 2 hours prior to surgery achieved good post-operative analgesia, decreased supplemental postoperative analgesic requirements and prolonged time to first rescue analgesic request with minimal side effects.
Recommendations
Recommendations are as follows;
- Use of gabapentin to improve the quality of post-operative pain control in our health institutions across the country as a component of multimodal analgesia regimen.
- Use of gabapentin as pre-operative regimen in most developing African countries where the availability and supply of opioids is erratic.
- Further studies on its usage for day case post-operative analgesia and for possible adoption as a premedication drug during surgery.
Conflict of interest
The Authors of this article declare that there is no conflict of interest and that no funding from any external source was received during performance of this study.
References
- Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms, 1994 2nded IASP Press, Seattle
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the peri-operative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management Anesthesiology 20121162248–273
- Royal College of Anaesthetists. Information for Patients (online). Available at: www.rcoa.ac.uk/clinicalstandards-and-quality/patient-information-leaflets (Accessed 21st September, 2017).
- Verma R, Alladi R, Jackson I, Johnston I, Kumar C, Page R, Smith I, Stocker M, Tickner C, Williams S, Young R. Day case and short stay surgery: 2. Anaesthesia. 2011; 66(5):417-34.
- Kulkarni S, Harsoor SS, Chandrasekar M, Bhaskar SB, Bapat J, Ramdas EK, Valecha UK, Pradhan AS, Swami AC. Consensus statement on anesthesia for day care surgeries. Indian journal of anaesthesia. 2017; 61(2):110.
- Beauregard L, Pomp A, Choiniere M. Severity and impact of pain after day-surgery. Can J Anaesth. 1998; 45: 304-311.
- Tong D, Chung F. Postoperative pain control in ambulatory surgery. Surg Clin N Am 1999; 79(2): 401-31.
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adiaka R, Sehgal N, Glaser SE, Vallejo R. Opioid Complications and Side effects. Pain Physician. 2008; 11:S105-S120
- Bonnefont J, Courade JP, Alloui A, Eschalier A. Antinociceptive mechanism of action of paracetamol. Drugs. 2003; 63:1 – 4.
- Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77: 362 – 379.
- Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: A meta-analysis. Br J Anaesth 2011; 106:454-462.
- Cheng JK Chiou L.C Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006; 100(5): 471-486
- Montazeri K, Kashefi P, Honarmand A. Pre-emptive gabapentin significantly reduces post-operative pain and morphine demand following lower limb extremity orthopaedic surgery. Singapore Med J. 2007; 48 (8): 748-751. (16)
- Bafna U, Verma AP, Rajarajeswaram K, Khandelwa M. A comparison of effect of preemptive use of oral gabapentin and pregabalin for acute post-operative pain after surgery under spinal anaesthesia. Journal Anaesthesiol Clin Pharmacol 2014; 30 (3): 373-377.
- Dirks J, Frendsborg BB, Christensen D, Fomsgaard JS, Flyger H, Dhal JB. A randomized study of the effects of single dose gabapentin versus placebo on post-operative pain and morphine consumption after mastectomy. Anesthesiology 2002; 97: 560-564
- Jaykaran C, Tamoghna B. How to calculate sample sizes for different study designs in medical research. Indian J Psychol Med.2013; 35 (2): 121-126.
- 17.Liza T, Peter F. Pain management in day case surgery: Continuing education in anesthesia, critical care and pain..2015; 15: 180-183.
- Mardani-Kivi M, Mobarakeh MK, Keyhani S, Motlagh KH, Ekhatiari KS. Is Gabapentin Effective on Pain Management after Arthroscopic Anterior Cruciate Ligament Reconstruction? A triple blinded randomized controlledtrial. Arch Bone Joint Surg. 2013; 1(1): 18-22.
- Luscombe KS, McDonnell NJ,Muchatuta NA, Peach MJ, Nathan EA. A randomized comparison of parecoxib versus placebo for pain management following minor day stay gynaecological surgery. Anaesthesia Intensive Care. 2010; 38 (1): 141-148.
- Aryal D, Gurung R, Marhatta MN. Evaluation of the effect of gabapentin on postoperative analgesia with epidural morphine after abdominal hysterectomy. JCMS Nepal 2017; 13 (2): 251-257.
- Butt A. Mohammad K. Ommid M, Jehan N, Qazi S. A randomized double blind placebo controlled study of prophylactic gabapentin for prevention of post-operative pain and morphine consumption in patients undergoing mastectomy. The Internet Journal Of Anaesthesiology.2010; 30:1
- Hema VR, Ramadas KT,Bigi KP, Indu S, Arun A. A prospective observational study to evaluate the role of gabapentin as preventive analgesic in thyroidectomy under general anesthesia. Anesthesia essays and researches. 2017; 11 (3): 718
- Rajendran I, Basavareddy A, Meher BR, Srinivasan S. Prospective, randomized, double blinded controlled trial of gabapentin and pregabalin as pre-emptive analgesia in patients undergoing lower abdominal and limb surgery under spinal anesthesia. Indian J Pain 2014; 28:155-159.
- Panah Khahi M, Yaghooti AA, Marashi SH, Nadjafi A. Effect of preemptive gabapentin on post-operative pain following lower extremity orthopedic surgery under spinal anesthesia. Singapore Med J. 2011: 52 (12): 879-882.
- Ebong EJ, Mato CN, Fyneface-Ogan S. Pre-Incisional Intravenous Low-Dose Ketamine Does Not Cause Pre-Emptive Analgesic Effect following Caesarean Section under Spinal Anaesthesia. J Anesthe Clinic Res. 2011; 2 (138): 2155-6148? 2158
- Jabalameli M, Hornamand A, Safavi M, Chitsav M. Treatment of post-operative nausea and vomiting after spinal anesthesia for Caesarean delivery: A randomized double blinded comparison of midazolam, ondansetron and a combination. Adv Biomed Res. 2012; 1: 2.
- Cruz FM, Taranto P et. al. Gabapentin for the prevention of chemotherapy-induced nausea and vomiting: A pilot study. Support Care Centre 2012; 20(3): 601-606
- John P, Cunha DO. Drug Centre-Rxlist-common side effects of neurotin (Gabapentin) http://www.rxlist.com/neurotin.siseeffect-drug-centre htm 10/17/2018.
- Turan A, Kaya G, Karamanlioglu B, Pamukccu Z, Apfel CC. Effect of oral gabapentin on post-operative epidural analgesia. Br J Anaesth 2006; 96: 242-246
- Parikh HG, Dash SK, Upasani CB. Study of the effect of oral gabapentin used as pre-emptive analgesia to attenuate post-operative pain in patients undergoing abdominal surgery under general anaesthesia. Saudi J Anaesth.2010; 4 (3): 137-141
- Hussain AM, Khan FA, Ahmed A, Chawla T, Azam SA. Effect of gender on pain perception and analgesic consumption in laparoscopic cholecystectomy: An observational study. J Anaesthesiol Clin Pharmacol. 2013; 29 (3): 337-341
- Amanor-Boadu SD, Sanusi AA, Abdullahi AA. Ketamine for preemptive analgesia in major gynaecological surgery. Nig J surg Res. 2003; 5 (1-2):7-11.
Download this article as PDF here: https://appconnect.daysurgeryuk.net/media/29258/ebirim.pdf