An audit of the perioperative complications of intrathecal baclofen and morphine pumps in Swansea (1998-2016) and a patient satisfaction survey « Contents
Cristina Cernei
Final year medical student at Swansea University Graduate Entry Medicine, Morriston Hospital, Swansea
Keywords: intrathecal, baclofen, morphine, pumps, rate, perioperative
Abstract:
Introduction: Intrathecal baclofen (ITB) and morphine (ITM) pumps improve pain, spasticity and QoL. Pump explanations due to complications are more expensive and traumatic for patients. Determining complication rates is beneficial from a bio-psychosocial aspect.
Aims: Determine the perioperative complications post-primary pump implantation (1998-2016); perform a service evaluation.
Methods: A retrospective review of indications, dose changes over time, and the complications 14 days post-operatively.
Results: 48 adult patients were included. The overall perioperative complication rate was 24%. 10% were pump related (4 catheter blocks, 1 pump failure), 14% non-pump related (1 CSF leak, 3 haematomas, 2 superficial infections). The mean initial and current ITM doses were 4.27mg/day and 10.4mg/day, and 120.61 mcg/day and 215mcg/day for ITB. Patients rated the service as excellent. 73.4% ranked their QoL improvement moderate (2-4/5) to excellent (5/5). Moderate symptom control satisfaction (3.70/5 (ITB), 3.82/5 (ITM)). Conclusion: These results are comparable to those from the NHNN and current literature (level III evidence). There is a gap between the number of patients eligible for pump implantation and procedures performed per annum. Reviews across the UK including patient surveys are required to set a standard of care and support adequate resource allocation for the continuation of this service.
Introduction
Pain and spasticity impact on all aspects of life: sleep, work, social relationships, family life, finances.3 Spasticity is an increase in muscle tone with speed of stretch.14 Inhibitory descending pathways from the brain cortex to the spinal cord are lost due to central nervous system pathologies. Pain can be related to cancerous and non-cancerous causes; e.g. chronic/failed back syndrome, fibromyalgia, chronic pain syndromes.2
Management options for spasticity include several drug classes administered either orally or intrathecally. Benzodiazepines act on the GABAA receptors in the spinal cord, exert an inhibitory effect and ease spasticity. Dose increments are needed over time leading to more side-effects (drowsiness, headache, weakness, dizziness and sedation). Baclofen (GABAB receptor agonist) crosses the blood-brain barrier and eases spasticity most efficiently with similar side-effects if taken orally. Dantrolene acts on the sarcoplasmic reticulum inhibiting release of calcium with comparable side-effects and hepatitis.2 For botulinum Toxin A the dose is related to ideal body weight. A limited number of muscle groups can be treated once every four to six months.24
Pain can also be treated with intrathecal medication, namely morphine. Morphine acts on the opioid receptors in the brain and spinal cord. Respiratory drive depression is the most dangerous side-effect when taken orally for chronic conditions. Intrathecal therapy dates back to the 19th century. August Bier injected himself and six other patients with cocaine in the subarachnoid space heralding a new era for spinal analgesia.17 An RCT found that intrathecal compared to conventional morphine delivery has an 85% and 71% success rate respectively.5 ITM also improves patients’ quality of life (QoL) and longevity with fewer side-effects.5,6,25,37
Need for intervention:
In the US over $100 billion/year are spent on pain management. 35 million people miss work due to pain and 83 million felt that pain interfered with their activities.17 Spasticity is associated with pain, fatigue, urinary dysfunction, poor sleep and mental health ailments. It also correlates with embarrassment, emotional distress, anxiety and depression.17,37 Intrathecal therapy is more beneficial but $17,317 more expensive than conventional therapy initially. However, the overall lifetime savings have been estimated at $3,111 per patient.5
Although side-effects are mostly avoided by direct administration of the drug intrathecally, pump implantation is not risk free. The median treatment cost per day for pump removal due to battery lifespan was $9.26 compared to $44.59 if explanted due to complications.3 Monitoring these complications via audits and service evaluations can improve patient satisfaction and future practice.
Aims
Perform an audit on the perioperative complications post-pump implantation. Compare the results with the set standard.
Define that being measured
The main measured outcomes are the perioperative complications rate post-primary pump implantation along with patient satisfaction with the service and their symptom control (See Fig.3). Secondary outcomes involve initial indications, starting and current dose of morphine and of baclofen.
Planning and Preparation
For future practice, it is fundamental to apply good medical practice principles throughout our work. One of the learning outcomes set was to ‘understand and have experience of the principles and methods of improvement, including audit, adverse incident reporting and quality improvement, and how to use the results of audit to improve practice’.11(23(e),p.28) For this purpose, the following plan was implemented:
1) Contact the clinical and educational supervisors and assess the need for ethical approval
2) Liaise with the Audit Department and register the audit to gain access to patients’ files
3) Review the files, anonymise patient identifiers and record the following:
● Indications
● Type of pump
● Initial and current drug dose
● Post-operative complications recorded in the first 14 days
4) Set a standard by reviewing the literature and existing guidelines
5) Compile and analyse all findings
6) Present results at the National ITB Forum
Standard set
There is no set gold standard.30,31 The overall perioperative complication rate derived from the literature is between 25-30%.24,6,8 Reported complications are generally divided into24:
1) Mechanical (pump related)
2) Pharmacological side-effects
3) Surgical (technique and pump implantation)
4) Patient specific (infection, comorbidities causing complications)
5) Refill complications
There has been an apparent decrease in the perioperative patient specific complications post-ITB/ITM pump implantation throughout the years.18,19,22 The reported infection rate fell from 28.7% in 1992 to 8.71% in 2014.19,22 Pump related complications (i.e. pump failure) rate was 9.8% in 199218 and 1% in 2014.22
Methods
A retrospective analysis was performed on the perioperative complications rate up to 14 days post primary intrathecal baclofen and morphine pump implantation (1998-2016). Indications for pump implantation and dose changes over time were also recorded.
The audit was registered with the audit department, Morriston Hospital. Ethical approval was sought. None was needed as per the local ABMU guidelines and the University requirements as this audit was within the scope of a service evaluation (Fig. 3a, Appendix 3).
Patients were identified using a surgical list provided by the neurosurgeon of all patients wit an implanted ITB/ITM pump under his care. This was recorded and password protected in Excel. The report contains no patient sensitive information. Simple descriptive statistics were used to analyse the data. A literature review was performed to discuss the findings. The search included the following key terms on PubMed and NICE Guidelines: complications AND intrathecal baclofen AND morphine AND pumps.
The National Hospital for Neurology and Neurosurgery (NHNN) was contacted to gain access to a similar audit conducted in 2016. Consent was granted to present these findings at the National ITB Forum and in the current report.
The patient survey was created with advice from the spinal nurse practitioner. A six-point questionnaire containing general and specific questions was sent to patients with ITM and ITB pumps (Fig.3). The completed forms were returned in a pre-paid envelope to the neurosurgeon’s office.
Results
Patients included in the audit and survey
77 patients with a primary pump implantation were identified. The Audit Department limited the eligibility to 50 (due to resource availability). 49 files were delivered of which one was excluded due to insufficient information. Thus, 48 patients were included of which 46 patients qualified for entering the service evaluation survey, two patients having died from non-pump related causes.
Patient demographics
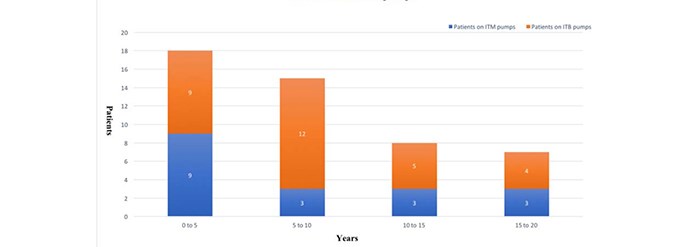
The study population is mainly comprised of adults, 13 Male and 35 Female patients. The average age was 48 years old. [2 sisters aged thirteen at the time of pump implantation in Nottingham were excluded from the average age data]. Patients have been on ITB and ITM pumps for an average of 8.7 and 8.4 years (Fig. 1).
Figure 1. Years on ITB/ITM pumps.
Indications
Overall the most common indication for ITB pumps was MS (57%) followed by acquired brain injury (17%) and hereditary spastic paraparesis (7%) (See Table 1.)
Table 1. ITB pump indications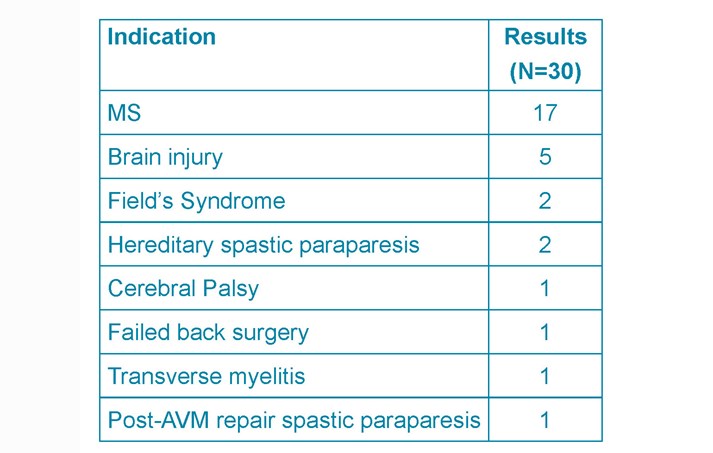
The most common cause for ITM pump implantation was chronic back pain (33%), followed by neurogenic pain (22%) and failed back syndrome (17%) (See Table 2).
Table 2. ITM pump indications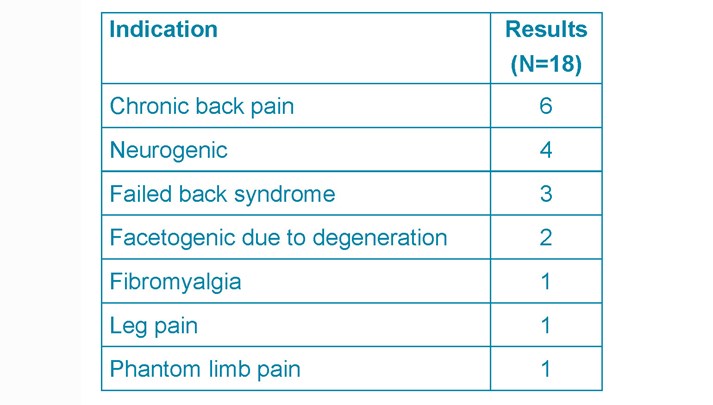
Perioperative complications
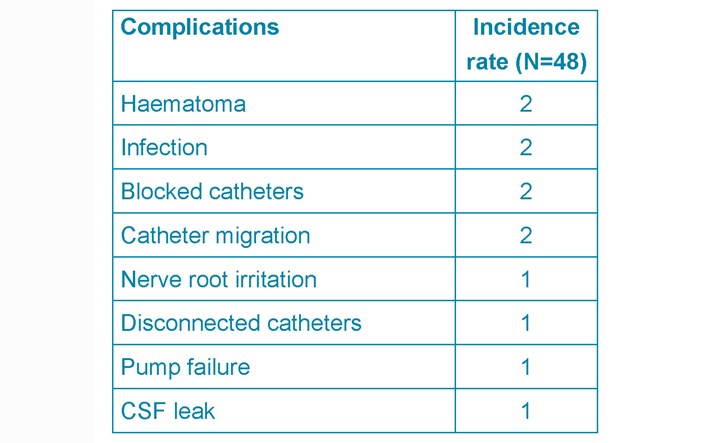
The overall perioperative complications rate was 25% (Table 3). These will be divided into three main groups:
- Infection rate
- mechanical complications
- pump system failure
Table 3. Summary of the perioperative complications
Infection rate
There were two superficial wound infections (4%) post-ITM pump implantation. In one the cultured organism was Staphylococcus epidermis, in the other case no organism was identified. Both were treated with IV cefuroxime, pump removal and re-implantation six months later. No comorbidities were recorded such as smoking, diabetes, obesity or positive MRSA status.
Mechanical complications
Mechanical complications comprise haematomas (4%), CSF leak (2%), catheter migration (4%). The first haematoma (ITB) case presented with discomfort at the site of pump implantation and was managed conservatively. The second (ITM) presented as an inflamed wound and subsequently diagnosed as an organised haematoma with no signs of infections.
One patient had two episodes of CSF leak with two different pumps following ITB pump implantation. This led to a pseudomeningocoele. Two patients had two and three episodes respectively of catheter migration. Both presented with leg pain and treated with pump revision and re-implantation.
Pump system complications
Pump system complications occurred in 8% of patients (3x ITB, 2x ITM): Pump failure (2%), disconnected catheter (2%), blocked catheter (4%). The issues were managed surgically with or without catheter readjustments.
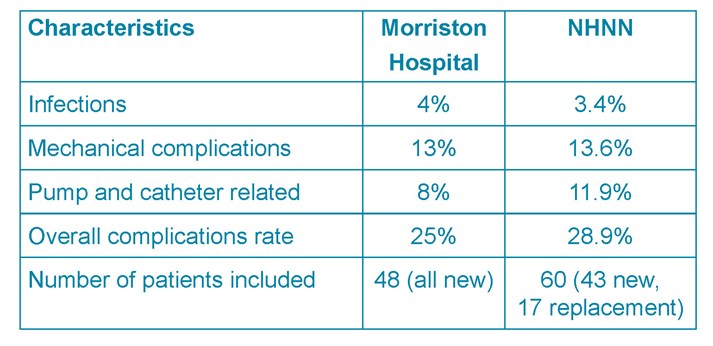
Table 4. Morriston hospital and NHNN results
Dose changes
The ITB and ITM dose is slowly titrated following pump implantation to achieve the balance of side-effects and improvement in patients’ QoL. The mean initial and current ITB dose for all indications was 120.61 mcg/day and 215mcg/day (Fig. 2; Table 1b, Appendix 2). Average initial and current dose for MS alone was 62.09 mcg/day and 138.5 mcg/day (Table 1.1a, Appendix 2). The modified Ashworth score recorded (13 out of 30 patients on ITB pumps due to MS) showed an average decrease of 1.53 points +/- SD 0.66 before and after baclofen trial (Table 1.1a).
Fig. 2. ITB and ITM dose increment trends
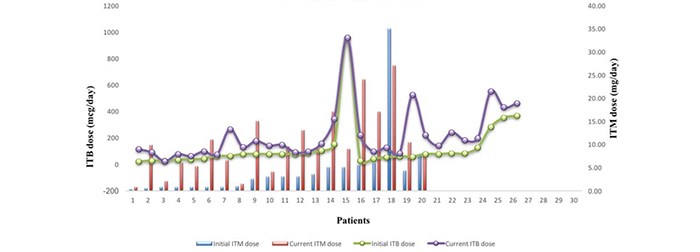
The mean ITM dose at discharge was 4.27mg/day and 10.4mg/day at the last refill recorded
(Fig. 2; Table 1.2a, Appendix 2).
The average current dose of ITM is 2.43 times greater than the discharge dose.
Patient survey
A simple six-point questionnaire was sent to 46 patients; 23 responses were recorded. Patients rated their answers on a scale from 1-5 (poor (1), good/moderate (2-4) and excellent (5)) or N/A (Not Available) if the question did not address their specific condition (i.e. if main indications were spasticity or pain alone). Patients’ names and age were included to monitor the number of responses returned (Fig. 3).
Fig. 3. Patient questionnaire

Patients’ suggestions and further commentaries to improve their experience were recorded in Table 5.2 (see Appendices).
Literature search
The search yielded 16 articles. 5 were excluded if the papers contained:
4. insufficient information
5. experts’ opinion and did not include complication rates
6. case reports with a study sample <10 patients.
A total of 11 studies were reviewed, mainly level III evidence (Table 6).
Discussion
Technique
Both ITB and ITM pumps were included in this analysis as the same technique and pump system (Synchromed II) is used. It consists of a catheter placed in the subarachnoid space via a spinal cannula in the lumbar region. A pump is placed subcutaneously in the left or right lower quadrant of the abdominal wall. 5,27,15,1 The drug of choice (morphine or baclofen) is injected through the skin in the filling port of the pump then delivered through the catheter.27 Subsequently the refill is typically every 2-3 months and pump replacement after approximately 5-7 years depending on the lifespan of the battery.
The aseptic technique involved skin preparation with alcoholic betadine followed by sterile sheets and iodine soaked swabs sewn to the skin edges. The pump system was bathed in gentamicin prior to implantation. The pocket for the pump was irrigated with gentamicin. Cefuroxime (1.5g IV) was given with induction of anaesthesia followed by 3 doses post-operatively (750mg IV 8-hrly).
Perioperative complications
The standard set is up to 25% according to the reported literature (see Table 6). This audit reports an overall complication rate of 25%. Similar results are identified by the NHNN (28.9%). Although marginally higher, their sample size and patient characteristics were slightly different (Table 4).
The rates of superficial wound infections between the hospitals were 4% vs. 3.4%. No serious cases such as meningitis or pocket infections were recorded in either series. However, pocket infections tend to develop over time and only the immediate perioperative period (14 days) was assessed33. From the literature an overall rate of 1.9%-12.3% for superficial, pump pocket, and catheter infections or meningitis is reported. 2,3,7,9,10,18,16,19,22,24,33 The two cases of superficial infections occurred post-ITM pump implantation. Patients on ITM for malignant causes of pain are considered at higher risk due to their immunocompromised states.30
Mechanical and pump and catheter complications were similar between Morriston and the NHNN series (13% and 13.6% vs 8% and 11.9%). The study of 100 cases in Sweden reported a 10.5% pump and catheter related complication rate, 4% infection rate involving the pump or catheter.10 Analysis of two case reports in a literature review report a 4.5% catheter and 3.8% pump related complications rate necessitating surgical revision.15 This amounts to 8.3% which is comparable to this audit’s data and to the NHNN. Again, the literature reports higher mechanical and pump system complication rates of up to 25% 2,3,9,10,18,16,19,22,24,33 and in one study nearly 75% were due to pump and catheter malfunction.7
Dose changes
An intrathecal baclofen trial is indicated prior to pump implantation.34 Muscle tone is assessed clinically pre- and post-trial using the Ashworth scores. An overall reduction of at least one point is a reliable indicator for successful spasticity management with intrathecal baclofen25, although others require a two-point decrease as an indication for pump implantation.27 Some centres do not undertake ITB trials at all26. The average decrease of 1.53 points +/- SD 0.66 recorded in the notes impacted on QoL enough to warrant long-term management using ITB pumps (Table 1.1a.).
The average difference in ITB dose change due to MS compared to other indications was 62.09mcg and 138.5mcg (Table 1.1a). A level III evidence recommended that cerebral compared to spinal origin for spasticity should be treated by dose increments of 5-15% vs. 10-30% every 24 hours.4 Others report the average baclofen dose for MS at 323mcg/day vs. 504mcg/day in patients with spasticity due to spinal-related causes.21 The dose tends to stabilise 12 months post-pump implantation7,21. A similar effect was detected in the current audit according to Fig. 2.
The mean current ITM dose is 2.43 times greater than the starting dose (Table 1.2.a, Appendix 2). In comparison, the respective value for baclofen is 1.78 times greater (Table 1b, Appendix 2). This trend is apparent in Fig. 2. Tolerance caused by opioid use is a well-known effect, less so for baclofen. ITM use up-regulates the expression of TAK1 (TGF-Beta Activated Kinase 1) protein in the dorsal horn of the spinal cord causing an attenuated analgesic effect35. Xu et al. propose the use of TAK1 inhibitors to reverse morphine tolerance.35 A drug holiday approach is also advisable using alternative drug for 4-6 weeks.21
Patient survey and recommendations
The NHS Commissioning Board states that ITB therapy should aim to prevent deformities caused by contractures and improve passive and active functioning.23,25,29 The survey attempted to assess patients’ physical function, muscle tone and QoL. 73.4% of patients ranked their QoL improvement as moderate (2-4) to excellent (5). Patients on ITM are less satisfied with their pain control than those on ITB (mean 3.67 and 4.24, SD 0.75 and 0.66 on morphine and baclofen respectively). This could further attest to the already recognised dose tolerance effect of morphine. Patients are moderately satisfied with their symptom control (averages 3.70 for baclofen and 3.82 for morphine). Similar findings are reported in a cross-sectional survey.20
Patients are very satisfied with the service and the pump itself while reporting mild-moderate improvement in their QoL. A cross-sectional multicentre study in Sweden observed the same effect.12 Patients attribute their inadequate symptom control or QoL to the already limiting condition, being bedbound or to a lengthy dose titration period.
Patients’ feedback and their suggested improvements considered:
- Lack of follow-up appointments and rehabilitation programs
- Lack of/low patient awareness regarding available pain management options
- Clinic location/availability
- Insufficient communication post-pump implantation (e.g. timeline relating to dose titration to achieve adequate symptom control, drug holiday approach for ITM users)
- Post-trial risks e.g. one patient developed infections on two occasions and claimed the last one to have culminated in a discectomy.
Most patients praised the neurosurgical team and the service itself, despite time constraints contributing to an ‘early discharge’ from consultant led follow-up clinics. The spinal nurse practitioner has been described as ‘incredible support’ and ‘always at the other end of the phone’.
Overall, patients deem the service as highly valuable. However, they need more support post-pump implantation (i.e. information about the procedure, expectations regarding treatment outcomes, physiotherapy). Similar findings have been reported by a pilot study that interviewed patients on ITB pumps.13 The service limitations identified are mainly due to financial insufficiencies impacting on most aspects of care. Only 3.1% of the NHS budget in Wales is spent on neurological disorders compared to 4.6% in England.28 In 2011 39,788 patients across the UK were waiting as outpatients for physiotherapy with waiting times between 1-40 weeks.28,32 Patients often opt for private services incurring further personal costs (Table 5.2). There is an already identified gap between demand and supply for intrathecal drug delivery systems implantation. The estimated need for ITB pumps is 4.6-5.7 per million population per annum based on incidence of eligible patients. The number of pumps implanted per annum in England during 2013-2014 were 3.08-4.38 per million.23
Conclusion and limitations
The audit process adhered to the hospital and university guidelines, and Tomorrow’s Doctors’ outcomes (e.g. maintain confidentiality, professionalism, teamwork)11. The results were within the standard set based on current available literature (a limitation to this analysis as it is mainly comprised of level III evidence). Multi-centre analysis (e.g. audits, RCTs) are needed to set a national standard. However, RCTs might be ethically and clinically difficult to arrange due to the severity the chronic conditions under investigation. Further, a dose tolerance effect was also observed in patients on ITM inferring from the results of the survey and the average increase in morphine dose. Patients are generally satisfied with the service provided and with the pump system. Although initially more expensive, ITB and ITM pumps are effective long-term from a bio-psycho-social perspective. Recommendations of this audit involve addressing the issues raised by patients. Insufficient resource allocation might be a limitation to implementing immediate changes.
Acknowledgements
Assistance from the clinical supervisor throughout the whole process was pivotal in the successful completion of this audit and service evaluation. Many thanks for their valuable input to the educational supervisor, spinal nurse practitioner, audit department, neurosurgeon’s secretary and to the NHNN for granting consent to use their results. Patients’ responsiveness in completing the service evaluation is also greatly appreciated.
References
- Abdulla S, Vielhaber S, Heinze H-J, Abdulla W. A new approach using high volume blood patch for prevention of post-dural puncture headache following intrathecal catheter pump exchange. International Journal of Critical Illness and Injury Science [Internet]. 2015 [cited 2018 January 05];5(2):93–8. Available from: http://www.ijciis.org/text.asp?2015/5/2/93/158395%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/26157652%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4477403
- Awaad Y, Rizk T, Siddiqui I, Roosen N, Mcintosh K, Waines GM. Complications of Intrathecal Baclofen Pump: Prevention and Cure. ISRN Neurol [Internet]. 2012 [cited 2018 January 05]; 2012:1–6. Available from: http://www.hindawi.com/journals/isrn/2012/575168/
- Bolash R, Udeh B, Saweris Y, Guirguis M, Dalton JE, Makarova N, et al. Longevity and cost of implantable intrathecal drug delivery systems for chronic pain management: a retrospective analysis of 365 patients. Neuromodulation Journal [Internet]. 2015 [cited 2018 January 05];18(2):150–6. Available from: doi: 10.1111/ner.12235.
- Boster AL, Adair RL, Gooch JL, Nelson MES, Toomer A, Urquidez J, et al. Best Practices for Intrathecal Baclofen Therapy: Dosing and Long-Term Management. Neuromodulation Journal [Internet]. 2016 [cited 2018 January 05];19(6):623–31. Available from: doi: 10.1111/ner.12388.
- Bottros MM, Christo PJ. Current perspectives on intrathecal drug delivery. Journal of Pain Research [Internet]. 2014 [cited 2018 January 06];7:615–26. Available from: doi: 10.2147/JPR.S37591
- British Pain Society. Intrathecal drug delivery systems for the management of pain and spasticity in adults; recommendations for best clinical practice [Internet]. 2015 [cited 2018 January 08]. Available from: https://www.britishpainsociety.org/static/uploads/resources/files/itdd_2015_pro_v3.pdf
- Draulans N, Vermeersch K, Degraeuwe B, Meurrens T, Peers K, Nuttin B, et al. Intrathecal baclofen in multiple sclerosis and spinal cord injury: Complications and long-term dosage evolution. Journal of Clinical Rehabilitation. 2013 [cited 2018 January 03];27(12):1137–43. Available from: doi: 10.1177/0269215513488607
- Duarte R, Raphael J, Eldabe S. Intrathecal drug delivery for the management of pain and spasticity in adults: An executive summary of the British Pain Society’s recommendations for best clinical practice. British Journal of Pain. 2016 [cited 2018 January 03];10(2): 67-69. Available from: doi: 10.1177/2049463715587747
- Follett KA, Naumann CP. A prospective study of catheter related complications of intrathecal drug delivery systems. Journal of Pain Symptom Management [Internet]. 2000[cited 2017 June 05]; 19(3): 209-215. Available from: https://doi.org/10.1016/S0885-3924(99)00153-0
- Fluckiger B, Knecht H, Grossmann S, Felleiter P. Device-related complications of long-term intrathecal drug therapy via implanted pumps. Spinal Cord Journal [Internet]. 2008 [cited 2018 January 07];46(9):639–43. Available from: doi: 10.1038/sc.2008.24.
- General Medical Council. Tomorrow’s Doctors - Outcomes and standards for undergraduate medical education [Internet]. 2009 [cited 2018 January 05];1–108. Available from: https://www.gmc-uk.org/Tomorrow_s_Doctors_1214.pdf_48905759.pdf
- Gunnarsson S, Alehagen S, Lemming D, Ertzgaard P, Ghaderi Berntsson S, Samuelsson K. Experiences from intrathecal baclofen treatment based on medical records and patient- and proxy-reported outcome: a multicentre study. Disability and Rehabilitation Journal [Internet]. 2018 [cited 2018 January 05]; 8:1-7. Available from: doi: 10.1080/09638288.2017.1419291
- Gunnarsson S, Samuelsson K. Patient experiences with intrathecal baclofen as a treatment for spasticity – a pilot study. Disability and Rehabilitation Journal [Internet]. 2015 [cited 2018 January 07];37(10):834-41. Available from: doi: 10.3109/09638288.2014.943844
- Guglielmino a, Sorbello M, Fazzio S, Zingale SF, Bucolo GE, Pittalà G, et al. Continuous intrathecal baclofen administration by a fully implantable electronic pump for severe spasticity treatment: our experience. Minerva Anestesiology [Internet]. 2006 [cited 2018 January 07];72:807–20. Available from: https://www.researchgate.net/profile/Massimiliano_Sorbello/publication/6788994_Continuous_intrathecal_baclofen_administration_by_a_fully_implantable_electronic_pump_for_severe_spasticity_treatment_Our_experience/links/5885409f92851c21ff4b1284/Continuous-intrathecal-baclofen-administration-by-a-fully-implantable-electronic-pump-for-severe-spasticity-treatment-Our-experience.pdf
- Health Quality Ontario, Ontario HQ. Intrathecal baclofen pump for spasticity: an evidence-based analysis. Ontario health technology assessment series [Internet]. 2005 [cited 2018 January 04];5:1-93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23074476%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3382401
- Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation [Internet]. 2001 [cited 2018 January 05];4(3):111–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22151655
- Knight KH, Brand FM, Mchaourab AS, Veneziano G. Implantable intrathecal pumps for chronic pain: highlights and updates. Croatian Medical Journal [Internet]. 2007 [cited 2018 January 09];48(1):22–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17309136%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2080496
- Kravitz HM, Corcos DM, Hansen G, Penn RD, Cartwright RD, Gianino J. Intathecal baclofen.Effects on nocturnal leg muscle spasticity. American Journal of Physical Medicine and rehabilitation. 1992; 71:48-52 cited in Health Quality Ontario, Ontario HQ. Intrathecal baclofen pump for spasticity: an evidence-based analysis. [Internet]. Vol. 5, Ontario health technology assessment series. 2005. 1-93 p. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23074476%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3382401
- Malheiro L, Gomes A, Barbosa P, Santos L, Sarmento A. Infectious complications of intrathecal drug administration systems for spasticity and chronic pain: 145 patients from a tertiary care center. Neuromodulation [Internet]. 2015 [cited 2018 January 05];18(5):421–6. Available from: doi: 10.1111/ner.12265
- Mathur SN, Chu SK, McCormick Z, Chang Chien GC, Marciniak CM. Long-term intrathecal baclofen: Outcomes after more than 10 years of treatment. American Academy of Physical Medicine and Rehabilitation [Internet]. 2014 [cited 2018 January 06];6(6):506–513.Available from: http://dx.doi.org/10.1016/j.pmrj.2013.12.005
- McIntyre A, Mays R, Mehta S, Janzen S, Townson A, Hsieh J, et al. Examining the effectiveness of intrathecal baclofen on spasticity in individuals with chronic spinal cord injury: A systematic review. Journal of Spinal Cord Medicine. 2014 [cited 2018 January 08];37(1):11–8. Available from: doi: 10.1179/2045772313y.0000000102
- Motta F, Antonello CE. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience. Journal of Pediatric Neurosurgery [Internet]. 2014 [cited 2018 January 05];13(3):301–6. Available from: http://thejns.org/doi/10.3171/2013.11.PEDS13253
- Narendran RC, Duarte R V., Valyi A, Eldabe S. The need for and provision of intrathecal baclofen therapy for the management of spasticity in England: An assessment of the Hospital Episode Statistics database. British Medical Journal Open [Internet]. 2015 [cited 2018 January 08];5(6):7p. Available from: 10.1136/bmjopen-2014-007517
- Natale M, D’Oria S, Nero VV, Squillante E, Gentile M, Rotondo M. Long-term effects of intrathecal baclofen in multiple sclerosis. Clinical Neurology and Neurosurgery Journal [Internet]. 2016 [cited 2018 January 06];143:121–5. Available from: http://dx.doi.org/10.1016/j.clineuro.2016.02.016
- NHS Commissioning Board [Internet]. Clinical Commissioning Policy: Intrathecal Baclofen. England: NHS Commissioning Board; 2013 [cited 2018 January 08]. Available from: https://www.england.nhs.uk/wp-content/uploads/2013/04/d04-p-c.pdf
- NHS England. Clinical Commissioning Policy: Intrathecal Pumps for Treatment of Severe Cancer Pain. D08/P/a. 2012 [cited 2018 January 06];p.1–27. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/d08pa-intrathecal-pumps-oct15.pdf
- Ontario Health Quality. Ontario Health Technology Assessment Series [Internet]. 2015 [cited 2018 January 05];16(8):1–83. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808717/
- Hopkins S. Getting the balance right : allocating resources for health and wellbeing. Annual report of the Director of Public Health for Cardiff and Vale [Internet]. 2012 [cited 2018 January 09]; Available from: http://www.cardiffandvaleuhb.wales.nhs.uk/sitesplus/documents/1143/CV%20DPH%20Annual%20Report%20ENGLISH%202012.pdf
- Saulino M, Ivanhoe CB, McGuire JR, Ridley B, Shilt JS, Boster AL. Best Practices for Intrathecal Baclofen Therapy: Patient Selection. Neuromodulation [Internet]. 2016 [cited 2018 January 05];19(6):607–15. Available from: doi: 10.1111/ner.12447.
- Smyth C, Ahmadzai N, Wentzell J, Pardoe A, Tse A, Nguyen T, et al. Intrathecal Analgesia for Chronic Refractory Pain: Current and Future Prospects. Drugs Journal [Internet]. 2015 [cited 2018 January 04];75(17):1957–80. Available from: 10.1007/s40265-015-0471-1
- Medtronic Limited. National ITB forum document [Internet]. 2006 [cited 2018 January 09]; UK:Medtronic Limited.
- Turner-Stokes L. Specialist neuro-rehabilitation services: providing for patients with complex rehabilitation needs. Specialist Neurorehabilitation Service Standards [Internet]. Updated 2015 [cited 2018 January 07];(10):1–18. Available from: https://www.bsrm.org.uk/downloads/specialised-neurorehabilitation-service-standards--7-30-4-2015-forweb.pdf
- Varhabhatla NC, Zuo Z. Rising Complication Rates after Intrathecal Catheter and Pump Placement in the Pediatric Population: Analysis of National Data Between 1997 and 2006. Pain Physician Journal [Internet]. 2012 [cited 2018 January 05];15(1):65–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22270739007/s10198-013-0537-5
- Wilkes D. Programmable intrathecal pumps for the management of chronic pain: Recommendations for improved efficiency. Journal of Pain Research [Internet]. 2014 [cited 2018 January 07];7:571–7. Available from: 10.2147/JPR.S46929
- Xu H, Xu T, Ma X, Jiang W. Involvement of neuronal TGF-alpha activated kinase 1 in the development of tolerance to morphine-induced antinociception in rat spinal cord. British Journal of Pharmacology [Internet]. 2015 [cited 2018 January 06];172(11):2892–904. Available from: DOI:10.1111/bph.13094
- Zheng S, He L, Yang X, Li X, Yang Z. Evaluation of intrathecal drug delivery system for intractable pain in advanced malignancies. Journal of Medicine [Internet]: 2017 [cited 2018 January 09]; 96(11): 1–5. Available from: DOI: 10.1097/MD.0000000000006354
- Zettle UK, Henze T, Essner U, Flachenecker P. Burden of disease with multiple sclerosis patients with spasticity in Germany: mobility improvement study (Move I). European Journal of Health Economics [Internet]. 2014 [cited 2018 January 05]; 15(9):953-966. Available from: doi: 10.1007/s10198-013-0537-5.
Download this article as PDF here: https://appconnect.daysurgeryuk.net/media/30732/292-cernei.pdf